Coronavirus (COVID-19) Update: FDA Takes Multiple Actions to Expand Use of Pfizer-BioNTech COVID-19 Vaccine | FDA

Moderna seeks FDA authorization for a second booster dose of its coronavirus vaccine for all adults - The Washington Post
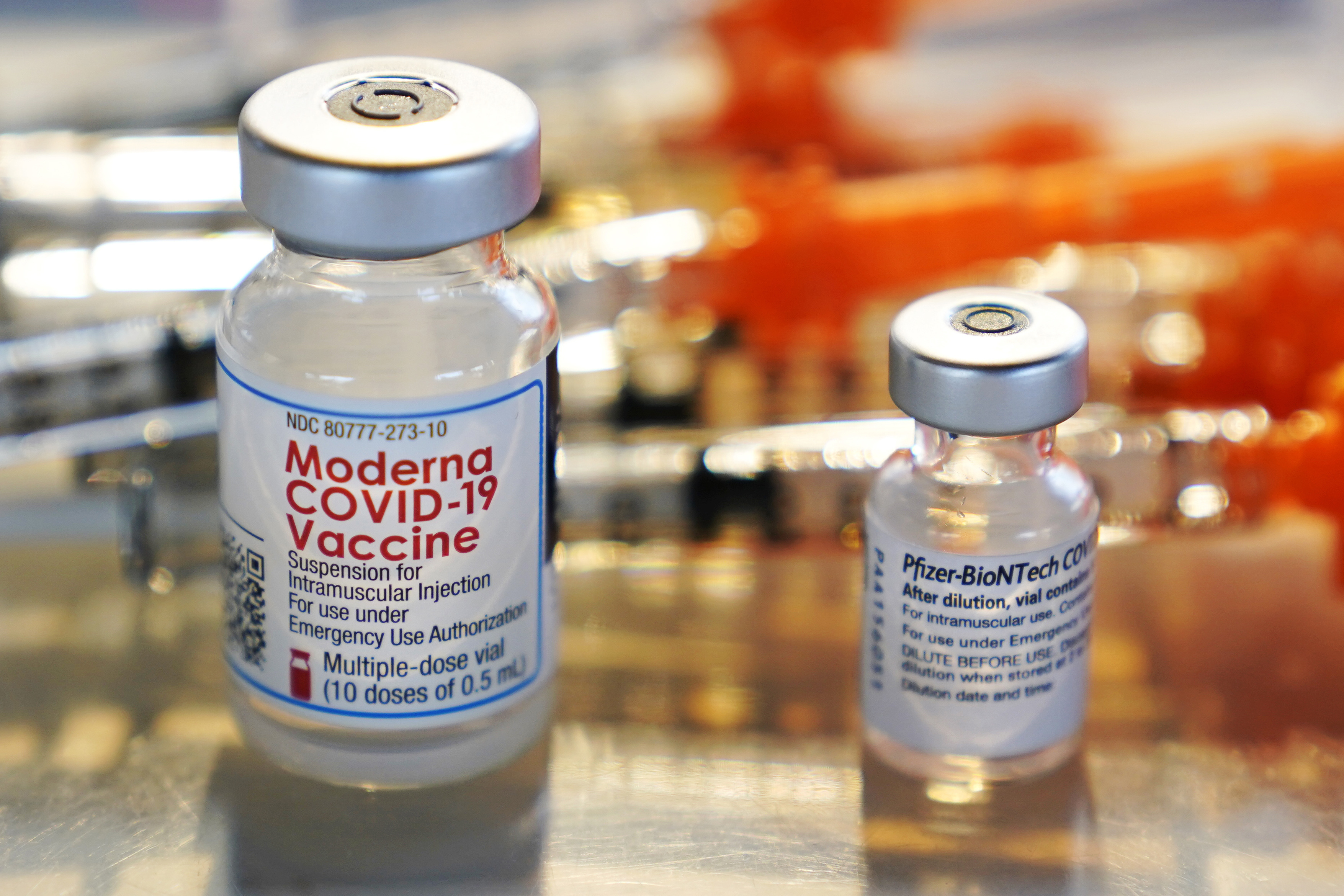
FDA authorizes another booster dose of the Pfizer or Moderna COVID-19 vaccine for people age 50 and up - The Boston Globe
FDA greenlights Pfizer booster shot for certain groups; CDC advisory panel votes to recommend boosters | AHA News

Omicron Subvariant Now Dominant in U.S.; FDA, CDC OK Second Booster for Adults Over 50 | Hartford HealthCare | CT

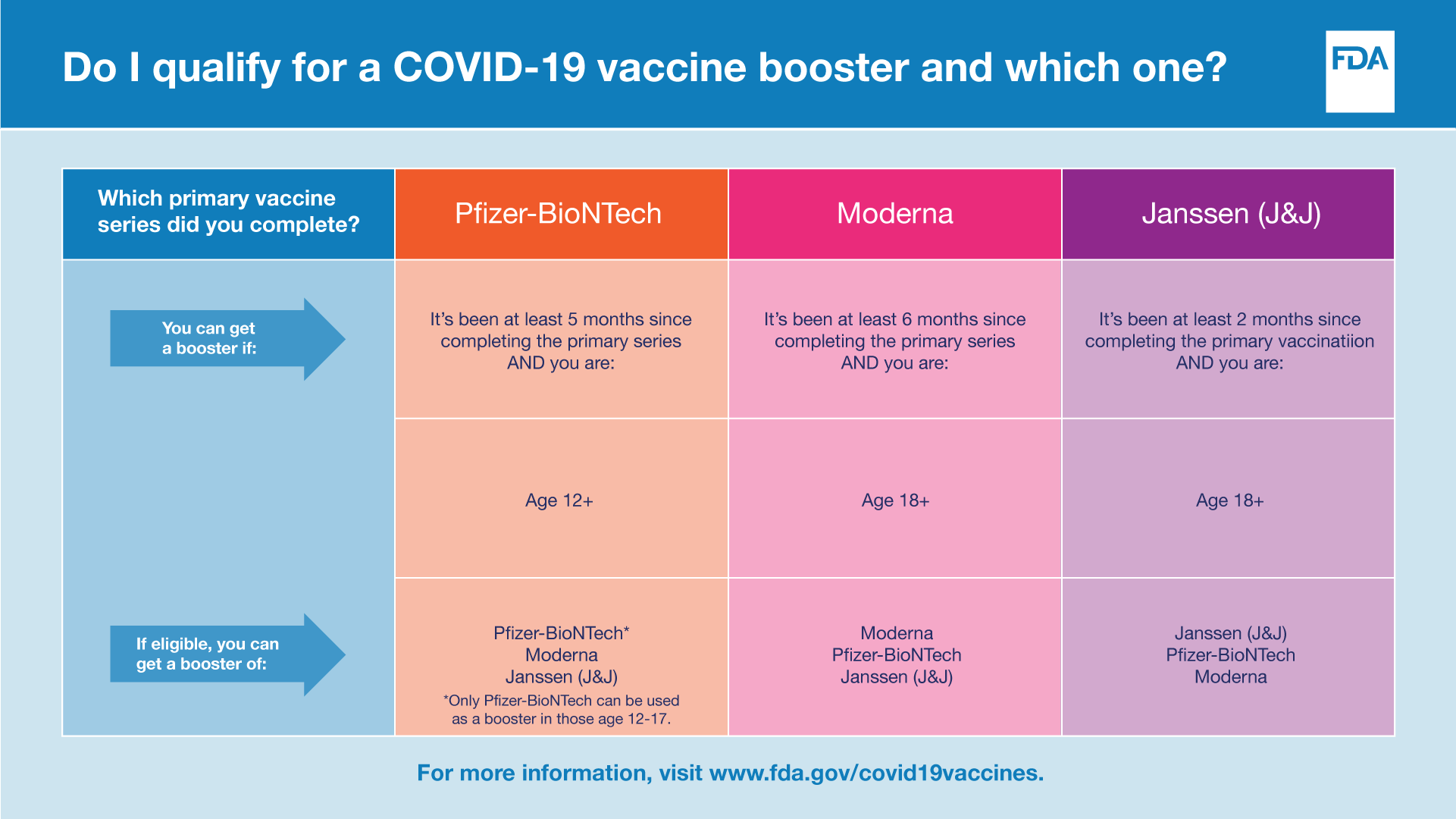
FDA authorizes second booster dose of two COVID-19 vaccines for ages 50+ and immunocompromised individuals







:max_bytes(150000):strip_icc()/Pfizer-and-Moderna-Is-Granted-EUA-For-Second-Booster-GettyImages-1229648268--2000-ce4026418a93431a97615e52868be276.jpg)